1. Introduction
Riparian zones are vegetational areas of an ecotone that separates upland and aquatic ecosystems. They typically occur along freshwater stream banks and serve as a buffer zone for sediment and nutrient runoff from uplands and floodplains draining into streams and rivers. By slowing down surface runoff, riparian vegetation serves as a vector for recharging groundwater and aquifers. Riparian vegetation features an abundant amount of compositional and structural biodiversity, occupying one of the most productive areas of the environment, increasing regional diversity by 50%
| [1] | Gregory, S. V., F. J. Swanson, W. A. McKee, and K. W. Cummins. 1991. An Ecosystem Perspective of Riparian Zones: Focus on links between land and water. BioScience 41(8), September 1991, 540–551. https://doi.org/10.2307/1311607 |
[1]
.
In our current era (CE), competition for wildlife habitat space in the United States is in a precipitous situation due to increasing demands for land development. Human population growth is in a steep, upward trend, with a projected 25.5 percent increase from 2016 to 2026—an increase of 81.4 million people just in the United States
. With this population growth comes the need to locate and shelter these people, and land development is right in step with demands for housing. The cause and effect of land development is loss of habitat, and the direct and indirect anthropogenic effects (e.g., erosion and excessive nutrient loading
| [2] | Peterjohn, W. T. and D. L. Correll. 1984. Nutrient Dynamics in an Agricultural Watershed: Observations on the role of a Riparian Forest. Ecology. 65; 5: 1466-75. https://doi.org/10.2307/1939127 |
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
[2, 3]
) on the earth’s ecosystems; namely, riparian habitats, which are one of the most biologically diverse and sensitive habitats on earth
| [2] | Peterjohn, W. T. and D. L. Correll. 1984. Nutrient Dynamics in an Agricultural Watershed: Observations on the role of a Riparian Forest. Ecology. 65; 5: 1466-75. https://doi.org/10.2307/1939127 |
| [4] | Stieger, M. and P. McKenzie. 2024. Riparian Landscape Change: A Spatial Approach for Quantifying Change and Development of a River Network Restoration Model. Environmental Management 74, 853–869 (2024). https://doi.org/10.1007/s00267-024-02025-w |
[2, 4]
. Introduction of exotic species into an ecosystem creates competition with the existing biota, resulting in loss of the native species. The effect from the introduction of exotic species adversely alters the way an ecosystem works, creating a more homogenous species dominance which directly alters the heterogeneity of species and consequent loss of biodiversity that alters the way an ecosystem was intended to work
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
[5]
. The local extinction of native and diverse species in an ecosystem caused by anthropogenically introduced exotic species is more common than global extinction, and this effect becomes irreversible
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
[5]
.
Diversity may be described as the variety of species and their relative abundance in an ecosystem
| [6] | Young, S. and L. N. Swiacki. 2006. “Surveying the Forest Biodiversity of Evansburg State Park: Plant Community Classification and Species Diversity Assessment.” International Journal of Botany, 2: 293-299. https://doi.org/10.3923/ijb.2006.293.299 |
[6]
. Particularly, the highly diverse structure of bottomland trees, shrubs, and vegetation found in riparian habitats, as they serve as a buffer zone by reducing sediment loads from surface runoff going into the stream
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
[3]
. Methods for quantifying ecological diversity have been well-documented (e.g.
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
| [38] | Gilbert, B. and J. M. Levine. 2017. Ecological drift and the distribution of species diversity. Proc. R. Soc. B 284: 20170507. https://doi.org/10.1098/rspb.2017.0507 |
| [39] | Nilsson, C., R. L. Brown, R. Jansson, and D. M. Merritt. 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 85, 837–858. https://doi.org/10.1111/j.1469-185X.2010. 00129.x |
| [40] | Hoppenreijs, J. H. T., L. Lind, and R. L. Eckstein. 2024. Effects of dispersal and geomorphology on riparian seed banks and vegetation in a boreal stream. J Veg Sci. 2024; 35: e13240. https://doi.org/10.1111/jvs.13240 |
| [41] | Wurzbacher, C., N. Wannicke, I. J. Grimmett, and F. Bärlocher. 2016. Effects of FPOM size and quality on aquatic heterotrophic bacteria. Limnologica 59 (2016) 109–115. https://doi.org/10.1016/j.limno.2016.04.001 |
[7-9, 37-41]
). Ways to measure diversity of a community is species richness, which is a measure of the health of an ecosystem, as well as maintaining the balance of hydrological cycles, energy input into freshwater ecosystems, and balancing the trophic pyramid
| [10] | Spiegelberger, T., D. Matthies, H. Müller-Schärer, U. Schaffner. 2006. Scale-dependent effects of land use on plant species richness of mountain grassland in the European Alps. Ecography 29: 541-548. https://doi.org/10.1111/j.0906-7590.2006. 04631.x |
[10]
.
In the United States, Texas is the second most biologically diverse state in the country
. A large number of Texas’ deciduous hardwood communities are found along riparian watercourses where there is an abundant supply of water. The increasing demand for land and water (which are limited resources) has raised the importance of riparian forests in terms of economic, aesthetic, and ecological factors. Economically, riparian forests maintain water quality by acting as a buffer zone for nutrient runoff into the watershed from farms and developed areas
| [2] | Peterjohn, W. T. and D. L. Correll. 1984. Nutrient Dynamics in an Agricultural Watershed: Observations on the role of a Riparian Forest. Ecology. 65; 5: 1466-75. https://doi.org/10.2307/1939127 |
[2]
. They also provide stability along the banks of streams and rivers and help prevent soil erosion
| [13] | Burandt, P., M. Grzybowski, K. Glińska-Lewczuk, W. Gotkiewicz, M. Szymańska-Walkiewicz, And K. Obolewski. 2024. Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains. Water 2024, 16, 164. https://doi.org/10.3390/w16010164 |
[13]
. Aesthetically, riparian forests are often scenic environments, providing an opportunity for people to enjoy unique species and habitat features (e.g. ecotourism
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
[5]
). Ecologically, these forests are complex communities, playing a vital role in the transfer of energy (trophic). They are often rich in habitat diversity, providing a niche for many plant and animal species
.
1.1. Denton Creek
Denton Creek is a third-order stream and a tributary of the Elm Fork of the Trinity River. From its headwaters, Denton Creek flows down to an impoundment, Grapevine Lake. From its spillway, Denton Creek flows past the study area in the City of Grapevine to its mouth at the confluence of the Trinity River. The study area lies within the Blackland Prairie Region of the Eastern Cross Timbers of north central Texas
. Vegetation along the banks of Denton Creek consists of facultative riparian (FAC) grasses, forbs, and hardwood trees that grow larger in height near the Transition Zone. The floodplain in this study consisted of grasses, woody shrubs, and a sparse mixture of honey mesquite and black willow that divided the study area into two separate stands of hardwood trees. The Transition Zone is where riparian trees transition to upland, i.e., from FAC riparian to facultative upland (FACU) and upland (UPL) trees and shade-tolerant grasses and forbs (
Table 1).
1.2. Facultative and Obligate Riparian Trees
Along the riparian corridor exists biologically rich interstices of woodlands separating stream and river ecosystems from upland ecosystems. Floodplains are associated with riparian watercourses and contain woody shrubs and trees that are classified as being both “obligate-riparian” and “facultative riparian.” Both categories play a significant role in stream bank stabilization, sediment and erosion control, assimilation of nutrients and pollutants, and provide a diverse habitat for specialized flora and fauna living within riparian habitats
| [15] | Rood, S. B., J. H. Braatne, and L. A. Goater. 2010. Responses of obligate versus facultative riparian shrubs following river damming. River. Res. Applic. 26(2) 102–117 (February 2010). https://doi.org/10.1002/rra.1246 |
[15]
. The ecological term ‘obligate’ suggests this category of woody shrubs and trees are restricted to wet soils in bottomland riparian zones. However, ‘facultative’ allows this category to exist in both riparian and upland areas. In bottomland riparian hardwood forests, the association of elms, ashes, and cottonwoods (a willow family tree) are typically found to exist in well-drained soils of the floodplain. In wetter soils along stream banks, the oak, gum, and cypress associations are usually found
. Examples of woody shrubs and trees found in Texas are shown in
Table 1.
Table 1.
Some woody shrubs and trees found in Texas riparian zones. Under the Wetland Indicator Categories are: Obligate Wetland (OBL), Facultative Wetland (FACW), Facultative Upland (FACU), and Obligate Wetland (UPL). Modification of Common Plants of Riparian Areas . | Woody | WI | Woody | WI |
Legend WI - Wetland Indicator Categories OBL Obligate Wetland Requires wet soil conditions and/or a high-water table. FACW Facultative Wetland Found in wet and seasonally moist soils FAC Facultative Can tolerate wet soil conditions as well as periodically dry conditions. FACU Facultative Upland Do not tolerate very wet soil conditions and are indicative of dry locations. UPL Obligate Upland Do not tolerate wet soil conditions and found away from riparian areas. | Buttonbush | OBL | Pecan | FAC |
Bald Cypress | OBL | Little Walnut | FAC |
False Indigo Amorpha sp. | OBL | Roosevelt Weed Baccharis sp. | FAC |
Black Willow | FACW | American Elder | FAC |
Arroyo Willow | FACW | Roughleaf Dogwood | FAC |
Green Ash | FACW | Pecan | FAC |
Spiny Aster | FACW | Red Mulberry | FACU |
Box Elder | FACW | Mesquite | FACU |
Possumhaw Ilex sp. | FACW | Black Walnut | FACU |
Salt Cedar | FACW | Netleaf Hackberry | FACU |
Sycamore | FAC | Mesquite | FACU |
Eastern Cottonwood | FAC | Western Soapberry | FACU |
American Elm | FAC | Bumelia | FACU |
Cedar Elm | FAC | Osage Orange | UPL |
Oaks | FAC | Juniper | UPL |
1.3. The River Continuum Concept and Sources of Nutrients in a Stream Ecosystem
Within a river ecosystem, the gradient of the drainage basin causes physical and biological dynamics driven by the topography and fluvial geomorphic processes; effectively regulating the energy / trophic input entering the stream
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
[7]
. The narrow headwaters of freshwater streams contain coarse sand, gravel, and cobble substrates and are shaded by riparian vegetation, supplying allochthonous input from senescing leaves. Due to the shading by trees, sunlight cannot provide the necessary photosynthetically available radiation (PAR) to induce primary production, i.e., chlorophyll-
a and phytoplankton
| [18] | Rolbiecki, D. 2024. Diel Vertical Migration by Copepoda, Cladocera, and Rotifera in Lake Texoma (Oklahoma-Texas). Journal of Environmental and Earth Science. 14(5) (2024). https://doi.org/10.7176/JEES/14-5-02 |
[18]
. Here the macroinvertebrates are primarily shredders and feed on the leaves and deposit coarse particulate organic matter (CPOM) for collector and grazer macroinvertebrates
| [19] | Rolbiecki, D. 2024. Diversity of Macroinvertebrate Communities Following Abnormal Spring Flooding of Denton Creek, Denton County, Texas. Corpus International Journal of Oceanography and Aquatic Research. 2(1). (2024). Article https://doi.org/10.54026/CIJOAR/1003 |
[19]
. At midstream, it widens to a point that allows some PAR for primary production, and in this area, CPOM is reduced to fine organic particulate matter (FPOM) where collector macroinvertebrate dominate the fauna. Along the widest part of the stream near its mouth, PAR is more prevalent to induce primary production of nutrients, whereby allochthonous input size is reduced and replaced by autochthonous organic matter in the form of dissolved organic matter (DOM)
| [40] | Hoppenreijs, J. H. T., L. Lind, and R. L. Eckstein. 2024. Effects of dispersal and geomorphology on riparian seed banks and vegetation in a boreal stream. J Veg Sci. 2024; 35: e13240. https://doi.org/10.1111/jvs.13240 |
[40]
; e.g., benthic chlorophyll-
a, where the dominant macroinvertebrates are collectors and filterers
| [19] | Rolbiecki, D. 2024. Diversity of Macroinvertebrate Communities Following Abnormal Spring Flooding of Denton Creek, Denton County, Texas. Corpus International Journal of Oceanography and Aquatic Research. 2(1). (2024). Article https://doi.org/10.54026/CIJOAR/1003 |
| [20] | Doretto, a., E. Piano, and C. E. Larson. 2020. The River Continuum Concept: lessons from the past and perspectives for the future. Can. J. Fish. Aquat. Sci. 77: 1853–1864(2020). https://doi.org/10.1139/cjfas-2020-0039 |
| [21] | Fenoglio, S., T. Bo, M. Cammarata, M. J. López-Rodríguez, and J. M. Tierno de Figueroa. 2014. Seasonal Variation of Allochthonous and Autochthonous Energy Inputs in an Alpine Stream”. Journal of Limnology 74(2). https://doi.org/10.4081/jlimnol.2014.1082 |
[19-21]
(
Figure 1).
Among the fauna affected by the input of organic matter made up from allochthonous deposits (e.g., seasonal fall senescing leaves), are the macroinvertebrates, which are a critical source of food resources for tertiary consumers at the apex of the trophic food pyramid
. Macroinvertebrates have evolved into specialized species adapted to feeding on CPOM, FPOM, and DOM. According to Vannote et al.
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
[7]
, riparian watercourses may be classified as 1) headwaters (First, Second, and Third Order), medium-sized streams (Fourth and Fifth Order), and large rivers (Sixth Order and greater). The primary food source input into First, Second, and Third-Order streams comes from allochthonous organic matter, e.g., leaf and grass litter
| [20] | Doretto, a., E. Piano, and C. E. Larson. 2020. The River Continuum Concept: lessons from the past and perspectives for the future. Can. J. Fish. Aquat. Sci. 77: 1853–1864(2020). https://doi.org/10.1139/cjfas-2020-0039 |
[20]
, which makes the diverse riparian areas of low-order streams extremely important, ecologically, in the overall trophic pyramid of freshwater streams (
Figure 1).
1.4. The Hyporheic Zone
Freshwater lakes and streams contain interstitial spaces where trophic transfer of energy takes place. In lentic (lake) systems, the interstices in the littoral zones are termed
psammon, which are various-sized sands and gravel surrounded by water spaces which allow benthic animals to thrive and feed on nutrients via gravitational and capillary action through these spaces. The types of benthos one would find include zooplankton feeding on phytoplankton, a primary producer in the trophic pyramid. In lotic (stream) systems, the mixture of coarse sand, gravel, and cobble substrate found in running water are located in the
rithron region of the stream. The heterotrophic input through the interstitial environment is known as the
hyporheic zone | [14] | Resh, V. H. and D. M. Rosenburg [Eds.]. 1984. The Ecology of Aquatic Insects. Praeger NYY. 1984. 625 p. https://doi.org/10.4319/lo.1984.29.6.1350 |
| [23] | Di Camillo, A. T., F. Cerasoli, M. Di Cicco, D. M. P. Maria Paola Galassi, and Tiziana Di Lorenzo. 2024. Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek. Diversity 2024, 16, 289. https://doi.org/10.3390/d16050289 |
[14, 23]
(
Figure 2).
1.5. Trophic Pyramid and Food Resources in Freshwater Streams
According to Resh and Rosenberg
and in a similar fashion from Lindman
, trophic relations in freshwater systems may be looked at as being five levels in a trophic pyramid: 1) primary producers (plants), 2) primary consumers (herbivores), 3) secondary consumers, 4) tertiary consumers, and 5) detritivores (decomposers) (
Table 2).
As illustrated in
Figure 1 and
Figure 2, allochthonous trophic inputs determined by stream order, riparian vegetation type and density, stream hydraulics and hydrology, and longitudinal stream gradient directly influence aquatic macroinvertebrates. Therefore, stream morphology and vegetation can predict the trophic status of freshwater streams
.
Table 3 summarizes the utilization of organic food resources by macroinvertebrates.
Table 2.
Trophic pyramid of aquatic macroinvertebrate functional feeding groups and trophic relations (modification of Resh and Rosenberg’s Table 6.2 ). Freshwater stream macroinvertebrates are influenced by allochthonous inputs of energy, which are directly associated with the density of riparian vegetation, stream morphology (discharge, width, gradient). These factors change as stream order increases, and the spatial ̶ temporal location and identity of stream macroinvertebrates are a good indicator of the trophic status in a particular reach of the stream . Macroinvertebrate Functional Feeding Group | Food and Feeding Mechanisms | Approximate Range of Food Particle Size (microns) |
Food | Feeding Mechanism |
Shredders | Living vascular hydrophyte tissue | Herbivores (chewers & miners) | > 103 |
| Decomposing leaves and wood (CPOM) | Detritivores (chewers, wood borers, gougers) |
Collectors | | Detritivores (filterers & suspension feeders |
| Decomposing FPOM | | < 103 |
| | Detritivores (gatherers & sediment feeders (incudes surface film feeders | |
Scrapers | Periphyton (attached to substrate cobble & wood) | Herbivores (grazers/scrapers off cobble and wood) | < 103 |
| Living vascular hydrophyte cell & tissue fluids / macroscopic algal cell fluids (DOM) | Herbivores (piercing tissue cells, sucks fluids | > 103—103 |
Piercers | Living animal tissue | Carnivores (attacks prey, pierces tissue & sucks fluids) | > 103 |
Predators | Living animal tissue | Carnivores (whole animals or parts) | > 103 |
1.6. Conservation and Management Best Practices
Due to the increased human population of North America and the large migration of out-of-state residents into the State of Texas, it is essential for programs of wildlife conservation practices to be on the forefront of local, state, and national authorities to implement more stringent regulations on land development in sensitive areas of rich biodiversity such as riparian ecosystems.
These practices would involve establishing the “protected status” of riparian corridors which would target land developers to abide by law restricting alteration of floodplains and bottomland habitats. A unified approach in ecological preservation can be the best way to achieving sustainable resources for the existing flora and fauna with the chance of preserving trophic input into riparian ecosystems which will continue to preserve species and biodiversity.
In urban areas such as the City of Grapevine where Denton Creek runs its course down to the Trinity River, urban conservation ordinances can be introduced to enable best practices in the sustainment of natural resources. One method would be the establishment of irrevocable and perpetual “Conservation Easements” of sensitive riparian ecosystems between their landowners and the local government. This would forever restrict land development in these biologically rich and diverse ecosystems. In doing so, benefits to the local economy may be achieved by establishing local or state wildlife parks, offering ecotourism, regulation of the harvesting of natural resources, and the enhancement of the commercial fishing guide industry, to name a few.
Table 3.
Food resources used by aquatic macroinvertebrates in headwater, mid-reach, and large rivers. (modification of Resh and Rosenberg’s Table 6.1 . In a given stream reach, nutrients in the particulates and dissolved organic matter are fundamental in the downstream flow of lotic ecosystems as they enter lentic ecosystems. Table legend: Nutrient resource type relative dominance: C = Common; S = Sparse; A = Absent. * = Conceptualized range of macroinvertebrate species richness. | Nutrient Resource Type |
Stream Order | Algae | Vascular plants | FPOM | Leaf litter CPOM) | Wood |
Headwater streams (orders 1-3) (100 – 250 species)* | S; some scraper species: Ephemeroptera Plecoptera Tricoptera Coleoptera Diptera | A (mosses & liverworts not included, but species using mosses: Ephemeroptera Tricoptera | C; many collector species: Ephemeroptera Tricoptera Diptera | C; many shredder species: Tricoptera Coleoptera Diptera | C; few shredder species: Tricoptera Coleoptera Diptera |
Mid-reach rivers (orders 3-6) (200-500 species)* | C; many species: Ephemeroptera Tricoptera Diptera | C; few species: Tricoptera Lepidoptera Coleoptera Diptera | C; many collector species: Ephemeroptera Tricoptera Coleoptera Diptera | S; few shredder species in protected areas seasonally or localized at entrance of low-order streams: Tricoptera Diptera | S to C; clumped distribution; few species: Diptera |
Large rivers (orders > 6) (10-50 species)* | S; very few species: Ephemeroptera Diptera | S to A; few if any; species: Diptera | C (during transport) (mostly Annelida, Mollusca); few species at high densities: Ephemeroptera Tricoptera Coleoptera Diptera | A (S in protected areas); shredders Rare or absent in Mid-reach rivers: Tricoptera Diptera | S to A; very clumped distribution; few if any species: wood burrowing Povilla spp. (Ephemeroptera) |
Downstream transport of nutrients into lentic systems is by
hydrochory, the process of dispersing organisms, plants and seeds by water
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
[37]
, and is often associated with
ecological drift . Through hydrochory, plant propagules are moved downstream in rivers—either by or long-distance dispersal, natural flooding, or anthropogenically-altered means such as damming or channelization—and deposited in riparian zones, whereby increasing species richness and plant colonization
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
[37]
. Ecological drift can be part of a stochastic, temporal change in a species or variation in fitness of a small population of organisms in an area or region, increasing the probability of niche differentiation, extinction, reproduction, and maturity. Ecological drift can also be a behavioral method of freshwater macroinvertebrates releasing themselves from the substrate to move downstream in order to avoid predation, or by dispersion of their offspring downstream. Flood disturbances (catastrophic drift) can also dislodge stream fauna further downstream
| [48] | Callisto, M., D. M. P. Castro, M. S. Linares, L. K. Carvalho, J. E. L. Barbosa, and R. M. Hughes. 2022. Which metrics drive macroinvertebrate drift in neotropical sky island streams? Water Biology and Security. https://doi.org/10.1016/j.watbs.2022.100077 |
[48]
.
Thus, from the aforementioned literature review, we can see that freshwater stream ecosystems are one of the most highly diverse biological communities on earth, amongst the equatorial tropical rainforests, marine coral reefs and estuaries, and coastal mangrove forests. They are extremely susceptible to natural disturbances—principally from abnormal flooding, as well as from anthropogenic influences through land development (followed by loss of habitat) which, in turn, natural pervious soils have now been paved, allowing for faster impervious surface runoff and the introduction of siltation and pollutants into this fragile ecosystem. There are also intentional (albeit without understanding the consequences thereof) anthropogenic stream and river alteration through channelization and damming. Other anthropogenic effects include the introduction of exotic flora and fauna (again, well-intended, but ignorant of the consequences), which, over time, outcompetes the native species and the eventual local extinction of these indigenous biota
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
[5]
. Since the introduction of the River Continuum Concept
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
[7]
, studies and research of lotic systems has predominantly been focused on the ecology and diversity of benthic macroinvertebrates near the headwaters (the most diverse area of the order of streams), and the local abiotic effects
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
[5]
from changes in stream hydrology (both natural and anthropogenic) affects the biodiversity of that site. However, there appears to be sparse research in regional patterns of biodiversity of the same and suggests a larger scale study (consider regional and larger areas) of lotic communities connected together (from upstream and downstream) and seeing the bigger picture of how they operate
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
| [25] | Tornwall, B., E. Sokol, J. Skelton, and B. L. Brown. 2015. Trends in Stream Biodiversity Research since the River Continuum Concept. Diversity 2015, 7, 16-35. https://doi.org/10.3390/d7010016 |
[8, 25]
and develop better practices for land use near riparian watercourses
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
[3]
.
2. Materials and Methods
This study was inspired following the completion of a boundary, topographical, and tree survey I performed by McCullah Surveying, Inc., in Addison, Texas from July-August 1999. The survey was under a contract with a civil engineering firm whose client was a land developer wanting to turn this ecologically sensitive area into a multi-family residential site along the south bank of Denton Creek. In addition to the other survey requirements, I recorded the location, description, and size of trees along the south bank of Denton Creek and provided our client the necessary information for planning purposes. The local regulatory authority, the City of Grapevine, Texas, required us to locate trees with a diameter at breast height (DBH) of three inches (7.62 cm) and above. The methods for quantifying diversity and community structure follows
Unit 5 in Brower et al.
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
[9]
Analysis of Communities, Community Structure, and Measures of Species Diversity.
2.1. Study Area
The study area is a stretch of bottomland riparian hardwood community in a floodplain located along the south bank of Denton Creek (centroid: 32°58'41.37"N // 097°02'21.25"W; elevation: 146m, see
Figure 3,
Figure 4), in the City of Grapevine, Tarrant County Texas, beginning at the west end of the F.M. 2499 (Grapevine Mills Parkway) bridge crossing, and extending west approximately 4,300 feet.
Drainage to the site comes from the south, running off a two-tiered plateau, beginning with a steeply sloped bank approximately 100 ft high (
30.48 m), flattening out to a wide (200 - 1000 ft [
61 – 305 m]) floodplain, and depositing off 25-foot (
7.62 m)-high banks into Denton Creek. A typical cross section of a stream or river consists of the “Toe Zone,” “Bank Zone,” Overbank Zone,” and the Transition Zone”
. The Toe Zone is located between the bed of the stream and the normal height of the surface of the stream. “
The bed is kept practically bare of upland vegetation by the wash of the waters of the rivers and is composed of light loose sand”
| [32] | Stiles A. 1924. The Gradient Boundary—The Line Between Texas and Oklahoma Along the Red River. Oklahoma v. Texas, 265 U.S. 500(1924). U.S. Supreme Court. (Reprinted in Texas Law Review, Volume 30 No. 3 in 1978. |
[32]
. It is the area usually devoid of upland vegetation when there is water, but in cases of drought conditions when the water level is low, grasses and sedges will grow above the low level of the water. The Bank Zone begins at the lowest qualifying bank (key bank) where bank full conditions of the stream first overtops the key bank
| [32] | Stiles A. 1924. The Gradient Boundary—The Line Between Texas and Oklahoma Along the Red River. Oklahoma v. Texas, 265 U.S. 500(1924). U.S. Supreme Court. (Reprinted in Texas Law Review, Volume 30 No. 3 in 1978. |
[32]
. There are usually backwashes at this location separating the key bank from higher ground
| [32] | Stiles A. 1924. The Gradient Boundary—The Line Between Texas and Oklahoma Along the Red River. Oklahoma v. Texas, 265 U.S. 500(1924). U.S. Supreme Court. (Reprinted in Texas Law Review, Volume 30 No. 3 in 1978. |
[32]
. Vegetation consists of grasses and short woody shrubs, and occasional willows, cottonwoods, and dogwoods. The Overbank Zone is located between the normal bank full level and the overbank elevation. This is normally the location of the floodplain.
Tree location was part of our client's requirements for a topographical survey to determine suitability for development of the site. Identification of trees was by learned knowledge of the species, or consulting Audubon’s “Field Guide to North American Trees”
| [28] | National Audubon Society. 1980. Field Guide to North American Trees. Eastern Region. Knopf Publishing Group, New York. 716 p. ISBN 9780394507606 |
[28]
. Specific requirements for locating trees were horizontal location, elevation, diameter at breast height DBH (3 inches [
7.62 cm] and above), and common name. If possible, their generic and specific names were noted. A “Spectra Precision Geodimeter 610” electronic total station theodolite was used to measure the location of trees and ground topography. DBH of trees was measured using a “Spencer Pro Tape” steel diameter tape measure read in inches.
The land surveying methodology required the establishment of local survey control by setting up the Geodimeter 610 on a tripod over a station whose three-dimensional coordinates have been previously established by Global Positioning System (GPS) static observations under an open, unobstructed sky, and precisely back sighting another previously established GPS station of known coordinates using a tripod with a prism set up over the back sight station. Distances were checked between the two survey control stations using a built-in electronic distance measuring (EDM) device via infrared readings to the back sight station. A traverse was started by fore sighting to another tripod with prism set up over a new survey control station inside the forest tree canopy and measuring the horizontal and zenith distance angles and EDM distances (GPS technology in 1999 did not have multipath error mitigation capability and was incapable of satellite observations under tree canopy). Then the Geodimeter 610 was carried forward and set up over the new control station and back sighting the previous survey control station; fore sighting to another survey control station set up under the tree canopy, and so on. The process of traversing through the tree canopy was carried out to allow enough survey control network densification to survey the topography and identified trees within the project area and was run all the way back to the initial GPS-derived back sight station, and the horizontal and zenith distance angles were turned to close back into the initial GPS-derived control station. The field traverse data was then downloaded into Trimble Terramodel software (Trimble, Inc., Westminster, Colorado), post-processed, and underwent a network adjustment. Following the traverse adjustment, field work in collecting topographical data and tying in identified trees was carried out using the station set up and back sight method, and fore sighting to a survey rod with prism attached whose rod height was known. This process is commonly known as a “radial survey.”
2.2. Analysis of Data
Data analysis consisted of enumeration, and placing species counts by their DBH on an ordinal scale into eleven size classes: 3-5, 6-8, 9-12, 13-15, 16-18, 19-21, 22-24, 25-28, 29-36, 37-44-, and forty-five-inches DBH and above, respectively (7.62-12.7, 15.24-20.32, 22.86-30.48, 33.02-38.1, 40.64-45.72, 48.26-53.34, 55.88-60.96, 63.5-71.12, 73.66-91.44, 93.98-111.76, and 114.3 cm DBH ↑). Species counts were totaled and ranked according to their abundance and relative importance in terms of its presence among the community. The most abundant species was assigned rank 1, the second most abundant assigned rank 2, and so on.
Measures of species diversity and community structure was done using Brillouin's index
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
[9]
:
H=
H=
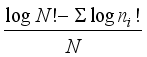 (1)
(1) where ni is the total count for species i and N is the grand total of all individuals.
Brillouin's index was chosen because tree samples were not random, but in effect the entire population of the community (sans < 3 inches (
7.62 cm) DBH). Species diversity is often used by ecologists as a measure of community stability
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[8]
. High diversity indicates a complex community, with a variety of interactions among organisms to include energy transfer, competition, and niche partitioning
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[8]
. The more diverse a community is, the more stable it becomes and resists environmental stresses
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[5, 8]
. A community is said to have high diversity if many species of equal abundance are present. On the other hand, a community having low diversity has very few species, or only a few species are abundant
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[5, 8]
.
Next, I compared my calculated index to a maximum value for diversity in the riparian community. The maximum possible diversity (H max) for N individuals in a total of s species occurs when the N individuals are distributed evenly among s species; i.e., when each ni = N ÷ s:
Hmax=
Hmax=
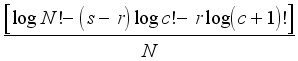 (2)
(2) where
c =
N ÷
s,
r = the remainder, i.e., the quotient of
N ÷
s is
c, and
r is the remainder
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
[9]
.
Using
H and
H max, the relative diversity of the community was determined by measuring evenness among species. Species evenness (
J) of the individuals' distribution among species is how close a set of recorded species abundances are from a collection of
N species having
H max | [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[8]
:
J=
J=
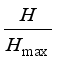 (3)
(3) Measures of dominance may be expressed by the quantity 1 –
J. Communities with low dominance will have low values with zero being the minimum; conversely, high dominance will approach 1, the maximum value for dominance
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
[9]
.
3. Results
25 species out of 14 families of trees were found in the Denton Creek study site. A total of 1,300 trees were located and recorded (See
Figure 5 and Appendix 1 and 2). The most abundant trees came from the elm family with a total of 769. Most species of elms live in water-rich soils and are often found in floodplains and riparian watercourses
| [29] | López-Almansa, J. C. 2004. Review. Reproductive ecology of riparian elms. Invest Agrar: Sist Recur For (2004) 13(1), 17-27 https://doi.org/10.5424/809 |
| [30] | Marks, C. O. 2017. The ecological role of American elm (Ulmus americana L.) in floodplain forests of northeastern North America. Proceedings of the American elm restoration workshop 2016. https://doi.org/10.273/NRS-GTR-P-174 |
[29, 30]
. Hackberry was the most abundant species of the elms followed by American Elm and Cedar Elm, respectively. Green Ash, the only species out of the olive family that was found at this site was third in order of species abundance (182 trees) and the second most dominant family. Elms and ashes are usually found together in bottomland riparian and floodplain habitats
| [28] | National Audubon Society. 1980. Field Guide to North American Trees. Eastern Region. Knopf Publishing Group, New York. 716 p. ISBN 9780394507606 |
| [29] | López-Almansa, J. C. 2004. Review. Reproductive ecology of riparian elms. Invest Agrar: Sist Recur For (2004) 13(1), 17-27 https://doi.org/10.5424/809 |
| [30] | Marks, C. O. 2017. The ecological role of American elm (Ulmus americana L.) in floodplain forests of northeastern North America. Proceedings of the American elm restoration workshop 2016. https://doi.org/10.273/NRS-GTR-P-174 |
| [31] | United States Department of Agriculture. 2020. Riparian Plants of Southern Texas.” Natural Resources Conservation Service Technical Note No: TX-PM-20-03. https://research.fs.usda.gov/treesearch/54950 Accessed 20241114. |
[28-31]
. The third most abundant family of trees was legumes (Redbud, Honey Mesquite, Honey Locust). Mesquite was found primarily in the open floodplain, and Locust was found on the bottom of the bank on the south tier, mostly in the lower, outer fringes of the tree stand. Mulberries (Red Mulberry and Osage Orange—vernacular Bois d' arc) were found in near-equal abundance, mostly along Denton Creek. Oak and Box Elder (beech and maple family, respectively) were found throughout both hardwood stands. The dominant oak species was Post Oak. There were 11 mature Post Oaks with a DBH between 22-24 inches (
55.88-60.96 cm). Oaks, ashes, and mulberries are well adapted to moist soil conditions and are typically found in riparian and floodplain communities
; see Appendix 1,
Table 5). Willows (Cottonwood and Black Willow) were found both in the floodplain and along the slopes of Denton Creek, where Cottonwood often exceeded 24 inches (
60.96 cm) DBH. The largest recorded Cottonwood at the site was 60 inches (
152.4 cm) DBH. Members of the walnut family (Pecan, Black Walnut) were found along the south side of Denton Creek. The majority of Pecan were mature specimens whose DBH was over 22 inches (
55.88 cm). Only one 16-inch (
40.64 cm) Black Walnut was found. By quick visual observation, many Black Walnuts and some Black Hickory (
Carya texana) were less than 3 inches (
7.62 cm) DBH—mostly as reproductive saplings, which were not surveyed. Sycamores (total 8 trees) were not plentiful as would be expected in a mixed, bottomland forest community, but one mature Sycamore was surveyed on the bank of Denton Creek whose DBH was 60 inches (
152.4 cm). A small number of non-dominating trees were found scattered throughout the community (Wooly Buckthorn, Hercules-club, Chinaberry, and Plum).
Table 4. Abundance and rank structure of the trees by their common name that were surveyed in 1999.
Species | Number of trees in each species | Rank |
Hackberry | 331 | 1 |
American Elm | 237 | 2 |
Ash | 182 | 3 |
Cedar Elm | 166 | 4 |
Box Elder | 47 | 5 |
Honey Mesquite | 46 | 6 |
Slippery Elm | 35 | 7 |
Red Mulberry | 34 | 8 |
Honey Locust | 34 | 9 |
Eastern Cottonwood | 31 | 10 |
Post Oak | 30 | 11 |
Osage Orange (Bois d' arc) | 28 | 12 |
Pecan | 24 | 13 |
Wooly Buckthorn | 11 | 14 |
Black Willow | 10 | 15 |
White Oak | 9 | 16 |
Hercules-club | 9 | 17 |
Sycamore | 8 | 18 |
Chinaberry | 8 | 19 |
Cedar (Ash Juniper) | 6 | 20 |
Plum | 6 | 21 |
Burr Oak | 5 | 22 |
Black Walnut | 1 | 23 |
Shumard (red) Oak | 1 | 24 |
Eastern Redbud | 1 | 25 |
Ranking species abundance among the community is shown in
Table 4. Hackberry was assigned rank 1 according to its dominance and its relative importance and influence among the community. Many birds including quail, woodpecker, and cedar waxwing eat its sweetish fruit
| [28] | National Audubon Society. 1980. Field Guide to North American Trees. Eastern Region. Knopf Publishing Group, New York. 716 p. ISBN 9780394507606 |
[28]
. American Elm, Green Ash, and Cedar Elm were assigned ranks 2,3,4, respectively. Red Mulberry, Post Oak, Osage Orange, and Pecan (ranks 8, 11, 12, 13, respectively) were of medium dominance and relative importance, however, their fruit provides food for birds and terrestrial animals. The least dominant species were Black Walnut, Shumard Oak, and Eastern Redbud (ranks 23, 24, 25, respectively). With the exception of the one Shumard Oak found, there were numerous sprigs of Walnut and Redbud under 1 inch (
2.54 cm) DBH found throughout the community.
Frequencies of trees in their respective family are presented in
Figure 6. Elms dominate all size classes, especially in the 3-5-, 6-8-, and 9–12-inch DBH classes (235, 255, 141, respectively). Green Ash was mostly found in the 3-5-, 6-8-, and 9–12-inch classes (44,33, 35, respectively). Mulberry and Osage Orange were found in the 3-5-, 6-8-, 9-12-, and 13–15-inch classes (23,23,14,2, respectively). Legumes (mesquite, locust, and redbud) were only found in the 3-5- and 6-8-inch classes (66, 14, respectively). Cumulative frequency curves of the most abundant tree families are presented in Appendix 2,
Figure 9.
Figure 6. Frequencies of trees by family with their DBH found at the Denton Creek study site.
Diversity among the 25 species found at Denton Creek was high (
Figure 7). Curve A in
Figure 7 takes on a horizontal aspect and shows relatively even abundance of each species in three rank groups: rank 1-4, rank 5-13, and rank 14-22. A community with high diversity will tend to have more species and an even abundance
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
| [6] | Young, S. and L. N. Swiacki. 2006. “Surveying the Forest Biodiversity of Evansburg State Park: Plant Community Classification and Species Diversity Assessment.” International Journal of Botany, 2: 293-299. https://doi.org/10.3923/ijb.2006.293.299 |
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[5, 6, 8]
. Curve B depicts a hypothetical community rich with species, and a perfectly even abundance, representing the highest diversity and lowest dominance. The chance of finding a situation such as Curve B in nature is virtually impossible.
Quantitative analysis of data of species diversity in this community supports the Curve B in
Figure 6. The value of Brillouin's index of diversity (
H) was 1.00 out of a maximum possible diversity (
H max) of 1.29. Relative diversity according to the evenness (
J) ratio of
H and
H max was 0.78, suggesting that this community is nearly 80% at its maximum possible diversity. Although the sample size of this community was large and considered being the sample population universe, the values of
H and
H max are probably underestimates since the survey was limited to sampling trees 3 inches (7.62 cm) DBH and above. Therefore, the value for evenness becomes an overestimate
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[8]
. The quantity of 1 –
J for measuring species dominance was low (0.22). This suggests that there is no one species in the Denton Creek study area exerting influence over the other species of trees from this study and is another indicator of the rich biodiversity within this community
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
[8]
.
4. Discussion
This study provided a pre-versus post-construction comparison of a sensitive and highly diverse riparian woodland community. It is now a multifamily apartment complex (
Figure 8). When the survey in 1999 was carried out, the study site—visually—appeared to be in near-pristine condition and representative of a model riparian habitat. The later construction of the apartments altered the natural floodplain topography, adding impervious surfaces such as asphalt and concrete pavement, and asphalt-shingled roofing. All of which increases surface runoff of rainwater, especially during unseasonal and abnormal flooding, which increases sediment loading into streams and rivers
| [4] | Stieger, M. and P. McKenzie. 2024. Riparian Landscape Change: A Spatial Approach for Quantifying Change and Development of a River Network Restoration Model. Environmental Management 74, 853–869 (2024). https://doi.org/10.1007/s00267-024-02025-w |
| [15] | Rood, S. B., J. H. Braatne, and L. A. Goater. 2010. Responses of obligate versus facultative riparian shrubs following river damming. River. Res. Applic. 26(2) 102–117 (February 2010). https://doi.org/10.1002/rra.1246 |
| [18] | Rolbiecki, D. 2024. Diel Vertical Migration by Copepoda, Cladocera, and Rotifera in Lake Texoma (Oklahoma-Texas). Journal of Environmental and Earth Science. 14(5) (2024). https://doi.org/10.7176/JEES/14-5-02 |
[4, 15, 18]
like Denton Creek. The anthropogenic effect from loss of habitat contributes to negative impacts on native riparian plants and trees from deleterious modification of hydrogeomorphology and sediment substrate
| [4] | Stieger, M. and P. McKenzie. 2024. Riparian Landscape Change: A Spatial Approach for Quantifying Change and Development of a River Network Restoration Model. Environmental Management 74, 853–869 (2024). https://doi.org/10.1007/s00267-024-02025-w |
| [15] | Rood, S. B., J. H. Braatne, and L. A. Goater. 2010. Responses of obligate versus facultative riparian shrubs following river damming. River. Res. Applic. 26(2) 102–117 (February 2010). https://doi.org/10.1002/rra.1246 |
| [33] | Brinson, M. 1993. A Hydrogeomorphic Classification of Wetlands. U.S. Army Corps of Engineers Waterways Experiment Station. Wetlands Research Program Technical Report WRP-DE-4. August 1993. Document location: 818514844.pdf |
[4, 15, 33]
.
In the research by Thornwall et al.
| [25] | Tornwall, B., E. Sokol, J. Skelton, and B. L. Brown. 2015. Trends in Stream Biodiversity Research since the River Continuum Concept. Diversity 2015, 7, 16-35. https://doi.org/10.3390/d7010016 |
[25]
, it was reported that studies in turnover and regional diversity (β- or γ-diversity) were sparse. Beta (β) diversity is the ratio between regional and local diversity. Gamma (γ) diversity represents the overall biodiversity of a larger geographic region. Specifically, the proportion of large-scale studies (i.e. “metacommunities”) which link the movement of “local” species from different communities together to influence population dynamics and community structure. Studies of stream diversity appear to be local-centric (e.g. local stream habitat, stream morphology, hydrological and disturbance variables), and intra- and inter-specific species interactions—when considered—are under reported in ecological literature. More studies aimed towards effective use of riparian buffer zones for the reduction of non-point source pollution into freshwater streams are needed to develop best management practices for land use near riparian watercourses.
Figure 8. Current Era post construction of this study of riparian tree biodiversity done in this study in 1999. The arrows point to the outer boundary of the area of the tree survey. The multifamily apartments and road networks have been constructed in the floodplain. Map image made from Google Earth aerial imagery.
Out of the most abundant tree family in this study, the hackberry was found to be the most dominant of the elms (
Table 1). Within the dense stand of the bottomland trees, the hackberry is a robust facultative riparian tree and has been reported to proliferate in this environment and can withstand periods of drought and fluvial disturbances
| [15] | Rood, S. B., J. H. Braatne, and L. A. Goater. 2010. Responses of obligate versus facultative riparian shrubs following river damming. River. Res. Applic. 26(2) 102–117 (February 2010). https://doi.org/10.1002/rra.1246 |
[15]
. The common misconception by the layperson that the hackberry is a so-called “trash tree,” nothing could be further from the truth. In fact, the common hackberry (
Celtis occidentalis) and its related family member the sugarberry (
C. laevigata) is a major food source for birds and provide habitat for other wildlife
| [46] | Wallace, M. C., W. B. Ballard, R. Swearingin, R. Walker, D. P. Holdstock, and B. Petersen. 2011. Rio Grande Turkey Diets in the Texas Panhandle. Proceedings of the National Wild Turkey Symposium 10: 201–211. |
[46]
. It is reported that the sugarberry-cedar elm-pecan forest is the most widely distributed makeup of bottomland trees in Texas
| [47] | Diamond, D., D. Riskind, and S. Orrzell. 1987 A framework for plant community classification and conservation in Texas. Texas Journal of Science 39: 203-221. |
[47]
. The elm-ash-maple-mulberry presence along the south bank of Denton Creek is indicative of co-dominant species tolerant of seasonal flooding in riparian bottomlands
.
The high presence of facultative riparian trees in this study plays a significant role in stream bank stabilization, a critical function of the floodplain, where this study took place
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
| [4] | Stieger, M. and P. McKenzie. 2024. Riparian Landscape Change: A Spatial Approach for Quantifying Change and Development of a River Network Restoration Model. Environmental Management 74, 853–869 (2024). https://doi.org/10.1007/s00267-024-02025-w |
| [16] | Anderson, S., and R. Masters. 2017. Water Quality Series-Riparian Forest Buffers. Oklahoma Cooperative Extension Service NREM-5034. https://extension.okstate.edu/fact-sheets/water-quality-series-riparian-forest-buffers.html Accessed 20241106. |
| [17] | Texas Riparian Association. 2015. Common Plants of Riparian Areas - East Central Texas. https://texasriparian.org/wp-content/uploads/2015/06/Riparian-Plants-East-Central-Tx-new.pdf Accessed 20241114. |
| [30] | Marks, C. O. 2017. The ecological role of American elm (Ulmus americana L.) in floodplain forests of northeastern North America. Proceedings of the American elm restoration workshop 2016. https://doi.org/10.273/NRS-GTR-P-174 |
| [31] | United States Department of Agriculture. 2020. Riparian Plants of Southern Texas.” Natural Resources Conservation Service Technical Note No: TX-PM-20-03. https://research.fs.usda.gov/treesearch/54950 Accessed 20241114. |
[3, 4, 16, 17, 30, 31]
(
Figure 8). Riparian flora allows for bank stabilization of streams and rivers by providing a buffer zone against erosion, sediment and nutrient loading into the water. The average bank-to-bank width of Denton Creek at this study site is 30 m, which can trap about 85% of sediments from polluting the water
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
| [34] | Clausen, J. C., K. Guillard, C. M. Sigmund, and K. n Dors. 2000. Water Quality Changes from Riparian Buffer Restoration in Connecticut. J. Environ. Qual. 2000, 29, 1751–1761. https://doi.org/10.2134/jeq2000.00472425002900060004x |
| [35] | Sweeney, B. W. and J. D. Newbold. 2014. Stream side forest buffer width needed to protect stream water quality, habitat, and organisms: A literature review. J. Am. Water Resour. Assoc. 2014, 50, 560–584. https://doi.org/10.1111/jawr.12203 |
| [36] | Ziegler, A. D., J. Negishi, R. C. Sidle, P. Preechapanya, R. A. Sutherland, T. W. Giambelluca, and S. Jaiaree. Reduction of Stream Sediment Concentration by a Riparian Buffer: Filtering of Road Runoff in Disturbed Headwater Basins of Montane Mainland Southeast Asia. J. Environ. Qual. 2006, 35, 151–162. https://doi.org/10.2134/jeq2005.0103 |
[3, 34, 35, 36]
. Sweeney and Newbold
| [35] | Sweeney, B. W. and J. D. Newbold. 2014. Stream side forest buffer width needed to protect stream water quality, habitat, and organisms: A literature review. J. Am. Water Resour. Assoc. 2014, 50, 560–584. https://doi.org/10.1111/jawr.12203 |
[35]
found in their review of the literature, protection of the physical, chemical, and biological integrity of small streams, buffer zones ≥ 30 m wide are needed. Although stream morphology was not the predominant requirement for the tree survey, its top banks on both sides were surveyed. In comparison to other study locations on Denton Creek
| [19] | Rolbiecki, D. 2024. Diversity of Macroinvertebrate Communities Following Abnormal Spring Flooding of Denton Creek, Denton County, Texas. Corpus International Journal of Oceanography and Aquatic Research. 2(1). (2024). Article https://doi.org/10.54026/CIJOAR/1003 |
[19]
, this stream width in this study site is wider (averaging 30 m) and appears to have significant allochthonous energy input and ability for PAR to take place in the production of phytoplankton.
The allochthonous and autochthonous trophic input of Denton Creek in the form of nutrients consisting of CPOM, FPOM, and DOM
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
[7]
is critical for food intake of primary, secondary, tertiary, and quaternary (detritivores)
. These nutrients are transported downstream into lentic systems via hydrochory, an important source of species colonization of recruitment-limited riparian–wetland communities which allows for the maintenance of the rich biodiversity of the communities’ biota
. This action also allows new communities of species to be established downstream far from the headwaters of streams and rivers. However, this does not account for the recruitment of riparian species at the headwaters since there is no dispersal mechanism from the stream’s beginning point. Dispersal of many plant species at the upstream (headwaters and tributaries) intercatchment via groundwater and rainfall runoff is made possible by guano from birds, soil and vegetation disturbances by large animals (e.g., livestock, deer), wind, and other means, and are thereby carried downstream to propagate
. Riparian communities are considered to be ‘local’ and distinct ecosystems amongst the landscape of larger, regional ecosystems, whereby the headwaters may have relatively high dominance of endemic plants (low diversity) due to fast-moving water, rocky substrate, lower plant nutrients, and colonized with specialized species of flora and aquatic fauna
| [42] | Sitati, A., M. J. Yegon, F. O. Masese, and W. Graf. 2024. Ecological importance of low-order streams to macroinvertebrate community composition in Afromontane headwater streams. Environmental and Sustainability Indicators. 21(2024) 100330. https://doi.org/10.1016/j.indic.2023.100330 |
[42]
. But through downstream ecological drift, plant species diversity increases within the community
. Within the riparian zone, the flow of water is cyclic, in that it reaches peak flow and peak exchanges during the rainy or monsoon seasons of spring, and decreases during the summer during low precipitation, and then in autumn when precipitation increases. Different species and functional feeding groups are contributors to downstream ecological drift—from headwaters and first and second-order tributaries—to higher-order mainstem streams by which in the vegetation and seed bank, species richness contributes to higher biodiversity
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
| [38] | Gilbert, B. and J. M. Levine. 2017. Ecological drift and the distribution of species diversity. Proc. R. Soc. B 284: 20170507. https://doi.org/10.1098/rspb.2017.0507 |
| [39] | Nilsson, C., R. L. Brown, R. Jansson, and D. M. Merritt. 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 85, 837–858. https://doi.org/10.1111/j.1469-185X.2010. 00129.x |
| [40] | Hoppenreijs, J. H. T., L. Lind, and R. L. Eckstein. 2024. Effects of dispersal and geomorphology on riparian seed banks and vegetation in a boreal stream. J Veg Sci. 2024; 35: e13240. https://doi.org/10.1111/jvs.13240 |
| [41] | Wurzbacher, C., N. Wannicke, I. J. Grimmett, and F. Bärlocher. 2016. Effects of FPOM size and quality on aquatic heterotrophic bacteria. Limnologica 59 (2016) 109–115. https://doi.org/10.1016/j.limno.2016.04.001 |
| [42] | Sitati, A., M. J. Yegon, F. O. Masese, and W. Graf. 2024. Ecological importance of low-order streams to macroinvertebrate community composition in Afromontane headwater streams. Environmental and Sustainability Indicators. 21(2024) 100330. https://doi.org/10.1016/j.indic.2023.100330 |
[37-41, 42]
. Therefore, this study supports the theory that hydrochory plays a significant role in plant colonization and species richness and diversity in riparian plant communities
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
[37]
.
The relatively high species diversity amongst the ranked groups of trees (
Figure 6) is consistent with other research
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
| [6] | Young, S. and L. N. Swiacki. 2006. “Surveying the Forest Biodiversity of Evansburg State Park: Plant Community Classification and Species Diversity Assessment.” International Journal of Botany, 2: 293-299. https://doi.org/10.3923/ijb.2006.293.299 |
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
| [8] | Brower, J. E., J. H. Zar, and C. N. von Ende. 1990. Field and Laboratory Methods for General Ecology. Third Edition. WCB Publishers, Dubuque. 273 p. |
| [9] | Zar, J. H. 1996. Biostatistical Analysis. Third Edition. Prentice Hall, New Jersey. 718 p. |
| [15] | Rood, S. B., J. H. Braatne, and L. A. Goater. 2010. Responses of obligate versus facultative riparian shrubs following river damming. River. Res. Applic. 26(2) 102–117 (February 2010). https://doi.org/10.1002/rra.1246 |
| [16] | Anderson, S., and R. Masters. 2017. Water Quality Series-Riparian Forest Buffers. Oklahoma Cooperative Extension Service NREM-5034. https://extension.okstate.edu/fact-sheets/water-quality-series-riparian-forest-buffers.html Accessed 20241106. |
| [37] | R. Jansson, U. Z. Ko, D. M. Merritt, and C. Nilsson. 2005. “Hydrochory increases riparian plant species richness: a comparison between a free-flowing and a regulated river.” Journal of Ecology 2005. 93, 1094 – 1103 https://doi.org/10.1111/j.1365-2745.2005.01057.x |
| [38] | Gilbert, B. and J. M. Levine. 2017. Ecological drift and the distribution of species diversity. Proc. R. Soc. B 284: 20170507. https://doi.org/10.1098/rspb.2017.0507 |
| [39] | Nilsson, C., R. L. Brown, R. Jansson, and D. M. Merritt. 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 85, 837–858. https://doi.org/10.1111/j.1469-185X.2010. 00129.x |
| [40] | Hoppenreijs, J. H. T., L. Lind, and R. L. Eckstein. 2024. Effects of dispersal and geomorphology on riparian seed banks and vegetation in a boreal stream. J Veg Sci. 2024; 35: e13240. https://doi.org/10.1111/jvs.13240 |
| [41] | Wurzbacher, C., N. Wannicke, I. J. Grimmett, and F. Bärlocher. 2016. Effects of FPOM size and quality on aquatic heterotrophic bacteria. Limnologica 59 (2016) 109–115. https://doi.org/10.1016/j.limno.2016.04.001 |
[5-9, 15, 16, 37-41]
. The study site at Denton Creek had an abundance of facultative wetland, facultative riparian, and facultative upland trees
, making this a functional creek in terms of dissipating the energy of stream flow and reducing floodwater flow, stabilizing banks, reducing erosion, trapping sediments that pollute waters downstream, creating floodplains and floodplain retention, and providing groundwater recharge. All of whom providing diverse wildlife habitats and increased water quality
| [2] | Peterjohn, W. T. and D. L. Correll. 1984. Nutrient Dynamics in an Agricultural Watershed: Observations on the role of a Riparian Forest. Ecology. 65; 5: 1466-75. https://doi.org/10.2307/1939127 |
| [3] | Liu, C., L. Qu, J. Clausen, T. Lei, and X. Yang. 2023. Impact of Riparian Buffer Zone Design on Surface Water Quality at the Watershed Scale, a Case Study in the Jinghe Watershed in China. Water 2023, 15, 2696. https://doi.org/10.3390/w15152696 |
| [4] | Stieger, M. and P. McKenzie. 2024. Riparian Landscape Change: A Spatial Approach for Quantifying Change and Development of a River Network Restoration Model. Environmental Management 74, 853–869 (2024). https://doi.org/10.1007/s00267-024-02025-w |
| [5] | Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer, and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75(1): 3-35. https://doi.org/10.1890/04-0922 |
| [6] | Young, S. and L. N. Swiacki. 2006. “Surveying the Forest Biodiversity of Evansburg State Park: Plant Community Classification and Species Diversity Assessment.” International Journal of Botany, 2: 293-299. https://doi.org/10.3923/ijb.2006.293.299 |
| [7] | Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Gushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37: 130-137. https://doi.org/10.1139/f80-017 |
| [10] | Spiegelberger, T., D. Matthies, H. Müller-Schärer, U. Schaffner. 2006. Scale-dependent effects of land use on plant species richness of mountain grassland in the European Alps. Ecography 29: 541-548. https://doi.org/10.1111/j.0906-7590.2006. 04631.x |
| [17] | Texas Riparian Association. 2015. Common Plants of Riparian Areas - East Central Texas. https://texasriparian.org/wp-content/uploads/2015/06/Riparian-Plants-East-Central-Tx-new.pdf Accessed 20241114. |
| [21] | Fenoglio, S., T. Bo, M. Cammarata, M. J. López-Rodríguez, and J. M. Tierno de Figueroa. 2014. Seasonal Variation of Allochthonous and Autochthonous Energy Inputs in an Alpine Stream”. Journal of Limnology 74(2). https://doi.org/10.4081/jlimnol.2014.1082 |
| [23] | Di Camillo, A. T., F. Cerasoli, M. Di Cicco, D. M. P. Maria Paola Galassi, and Tiziana Di Lorenzo. 2024. Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek. Diversity 2024, 16, 289. https://doi.org/10.3390/d16050289 |
| [43] | Pusey, B. J. and A. H. Arthington. 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54(1) 1–16. https://doi.org/10.1071/MF02041 |
[2-7, 10, 17, 21, 23, 43]
. However, as shown in this study, anthropogenic changes to freshwater streams and rivers makes them highly vulnerable through the alteration of floodplains, reducing the natural composition of the stream ecosystem by isolating flora and fauna populations and their habitats in the riparian community
| [44] | Brendenhal, E. and M. J. Samways. 2008. Impact of a dam on benthic macroinvertebrates in a small river in a biodiversity hotspot: Cape Floristic Region, South Africa. J Insect Conserv. (2009) 13: 297-307. https://doi.org/10.1007/s10841-008-9173-2 |
[44]
.
If we evaluate Denton Creek as a valuable resource for both human and ecological uses, these data suggest this community to be of high quality and a valuable and important asset to this geographical region. In terms of ecological importance, this riparian hardwood community is rich in habitat diversity that provides vegetative and protective cover for both flora and fauna, habitat niche, breeding sites and plant distribution, to name a few. During the 1999 survey, a family of about 12 North American Wild Turkey (
Meleagris gallopavo, a native upland bird of Texas) were seen along the outer fringes of the upland tree line. Texas boasts the greatest population of these upland birds; however, some research has shown that the American Wild Turkey population is declining due to habitat loss, e.g.
. In terms of human importance, the site has economic importance, both as a source of crop and domestic animal production, erosion control, water conservation, and land value. Human legal factors to consider in the development of this tract of land are regulations concerning water quality, wetland mitigation, endangered and protected species, and applicable zoning laws. In terms of aesthetic value, one might consider this site to be priceless, having unique features such as a scenic environment, diverse plant and animal species, or historical importance.
5. Conclusions
In this study, It is shown how the riparian community plays an important role in the ecological functions of flood control, bank stabilization, filters and buffers of pollution into the water, provisions for wildlife habitat, nutrient cycling by way of capturing and storing nutrients from the surrounding topography, and as a corridor for movement between different ecosystems. The River Continuum Concept was discussed in detail as the important link between lotic and lentic systems. The etymology of lentic comes from the Latin word
Lentus, meaning “calm” or “still”. The Latin word for lotic is
Lotus, which means “to wash”, e.g. fast-moving freshwater. Lotic systems include rivers, streams and creeks and unlike lentic systems, lotic systems contain higher species diversity in their communities. The trees that were surveyed along Denton Creek in 1999 were aggregated into taxonomical and diameter size classes, demonstrating that this area was found to be a highly diverse community of riparian woodlands. Since this study in 1999, the area has been developed into a multi-family apartment complex that threatens the ecological structure of Denton Creek; and is likely to have negatively impacted downstream community ecosystems, causing irreversible changes in land use, biodiversity, and the entire health of the river system of the Elm Fork of the Trinity River. Although it has been 25 years since the 1999 tree survey, it is not known if any field studies have been done on the urban affects on this riparian community directly related to best management practices of riparian aquatic flora and fauna
. As land continues to become urbanized, profound effects on biodiversity, community structure, and species richness. Urban landscapes tend to have lower biodiversity than pristine ecosystems
| [49] | Prescott, V. A. and P. K. Eason. 2018. Lentic and lotic odonate communities and the factors that influence them in urban versus rural landscapes. Urban Ecosystems (2018) 21: 737-750. https://doi.org/10.1007/s11252-018-0752-z |
[49]
. This study is concluded to bring about awareness of land development affecting the sensitive riparian ecosystem by implementing conservation and management best practices such as
Streamside Management Zones (SMZ) to buffer forestland adjacent to stream in the way of bank stabilization and the restriction of sediment and other pollution loads
| [51] | Schilling, E. B., A. L. Larsen‐Gray, and D. A. Miller. 2021. Forestry Best Management Practices and Conservation of Aquatic Systems in the Southeastern United States. Water 2021, 13, 2611. https://doi.org/10.3390/w13192611 |
| [52] | Na Du1, A. M. Fathollahi‑Fard, and K. Y. Wong. 2023. Wildlife resource conservation and utilization for achieving sustainable development in China: main barriers and problem identification. Environmental Science and Pollution Research. https://doi.org/0.1007/s11356-023-26982-7 |
| [53] | Abraham, A. J., E. S. Duvall, E. le Roux, A. Ganswindt, M. Clauss, C. E. Doughty, and A. B. Webster. Anthropogenic supply of nutrients in a wildlife reserve may compromise conservation success. Biological Conservation 284(2023) 110149. https://doi.org/10.1016/j.biocon.2023.110149 |
[51-53]
into sensitive riparian ecosystems in hopes of fostering proper land stewardship by promoting sustainable land strategies through the implementation of best management practices at the local, county, and state levels of natural resources regulation.
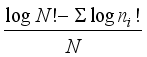 (1)
(1) 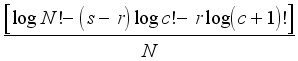 (2)
(2) 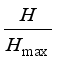 (3)
(3)